Main Body
2 WHAT PHOTONS ARE
p. 2
simple answer: traveling packets of vibrating energy
Electromagnetic radiation is emitted by all matter above absolute zero (0o Kelvin or -273o Celsius).
It is streams of energy bundles – photons.
A photon’s energy depends on its frequency of vibration:
Energy = frequency x Planck’s constant
Planck’s constant is 6.625 x 10-34 joule-second). It is the smallest amount of power possible.
Therefore photons only come in discrete (quantal) steps.
Multiplying their frequency in vibrations per second by Planck’s joule-seconds, cancels the
seconds, leaving Energy as joules – the unit of measurement for pure energy.
N.B. This is the only equation – except for one on page 5, 6, on 17 & 18, 25, and in a Figure on page 35 – no calculus. They’re only to show that such matters can be calculated. You don’t need to use them to get the point.
p. 3
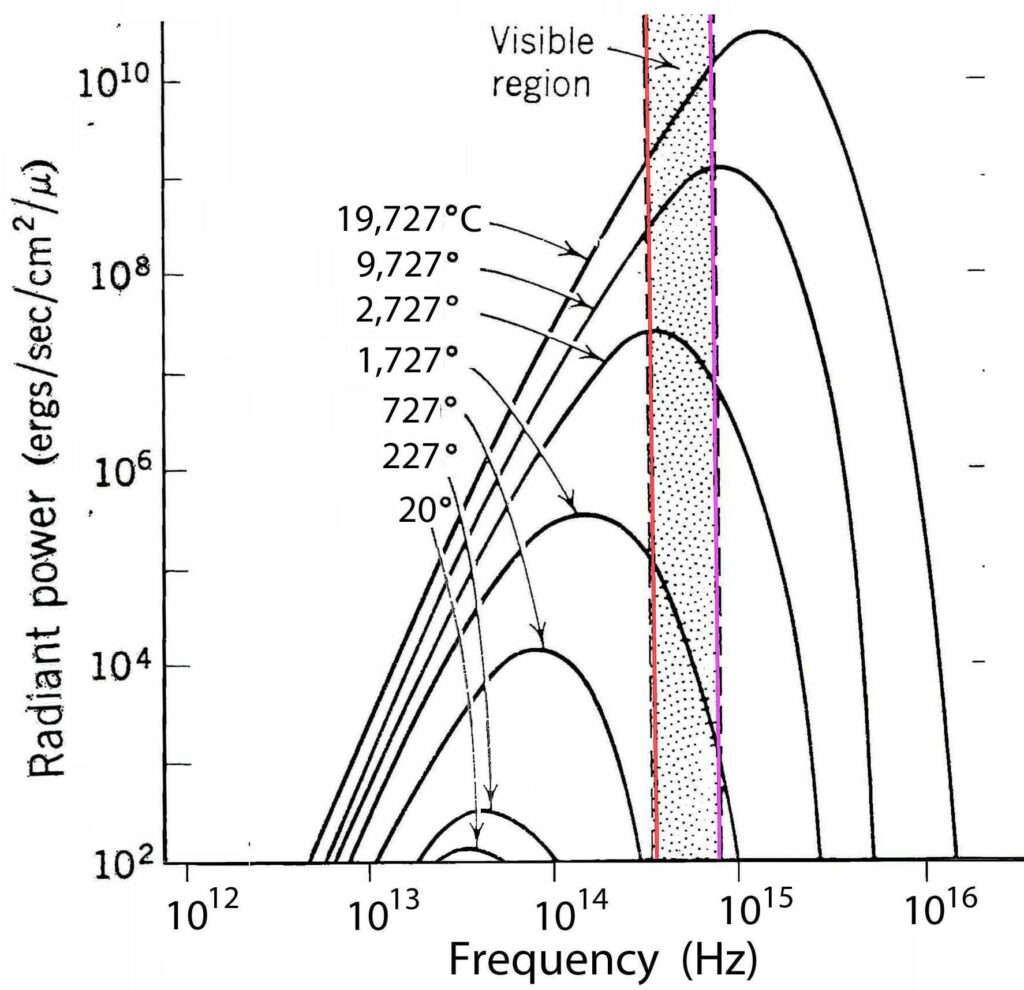
It takes more energy for matter to release higher frequency photons. Therefore, as temperature increases, more high frequency photons are emitted:
To picture the relationship between energy, frequency and wavelength, consider this: It takes more effort to shake a rope, carpet runner or bed sheet faster thereby increasing how many waves occur.
For ideal matter, the relation between temperature and energy of the emitted photons is called the Black Body Radiation Law:
It’s only an average. Real matter deviates from this ideal, but it’s a good place to start.
At room temperature, a square meter of ordinary stuff (stairs, spoons, etc.) emits one photon in about every 42 seconds – virtually none of them visible.2 That’s why we need lamps – even if we turn up the thermostat.
Photons vibrate transversely to their direction of travel. This results in sinusoidal-like envelops of energy around each photon’s path:
p. 4
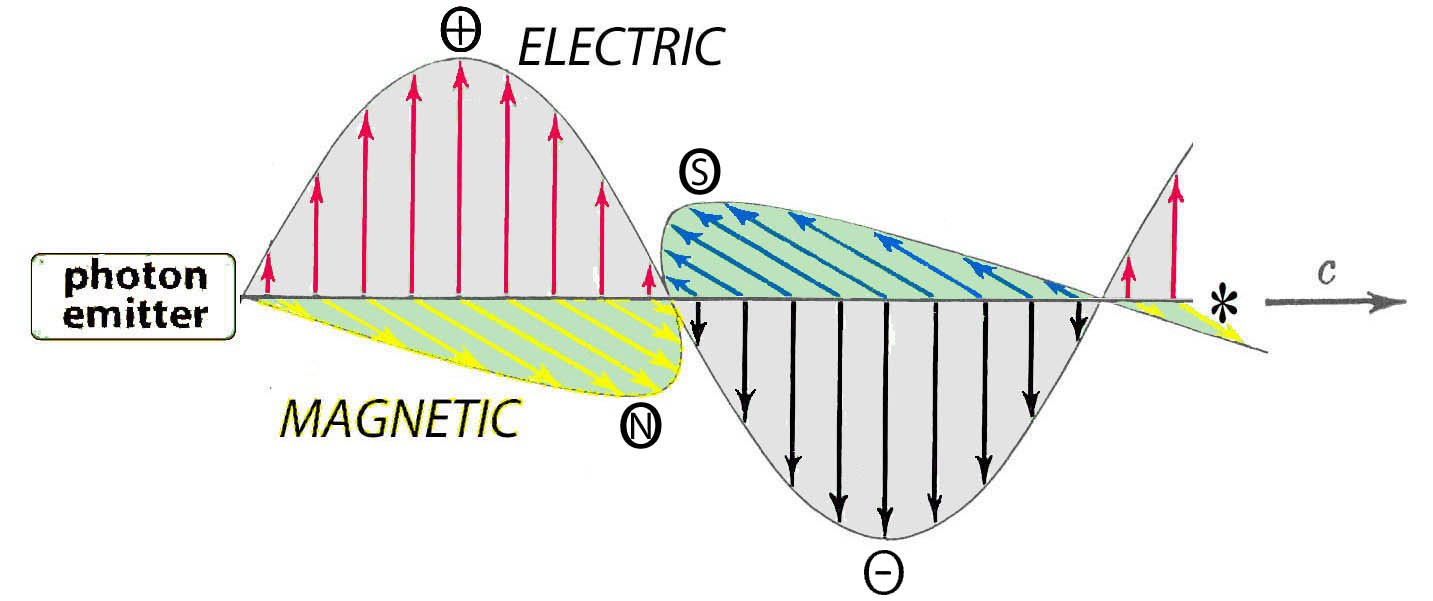
Their vibration expresses two kinds of energy: electric and magnetic
energy: electric and magnetic
It is a stable mutually reinforcing association:
The varying electric field induces a corresponding varying magnetic field at a right angle to itself, The varying magnetic field similarly induces a varying electric field at a right angle to itself.
Having no mass and vibrating so fast is why photons for many purposes can be successfully treated as traveling along the average location of their combined electric and magnetic fields.
For most lighting, chemistry, and vision work, attending to the electric wave is sufficient. So to simplify this glance, the magnetic component will not be discussed further.
p. 5
The speed of a photon depends on the medium through which it travels.
(Shortly, we shall learn why this is very fortunate.)
Photons travel 300,000,000 meters per second in vacuum and nearly as fast in air. This speed is generally called “c”.
n = c / speed in medium
For example:Photons entering glass (n = 1.5) slow down to:
speedglass = 3 x 108 m/s/ 1.5 = 2 x 108 m/s.
Why? Their vibrating electric field is hindered by fields of the electrons in the medium – an effect I’ll call “drag”. Denser media, tend to have stronger fields, larger refractive indices and exert more drag.
Traveling in the opposite direction from right to left above, the photons are released from the drag and resume their speed and wavelength.
p. 6
The distance a photon has traveled during one complete cycle of vibration is called its “wavelength”. A photon’s wavelength therefore depends on its velocity:
wavelength = speed / frequency
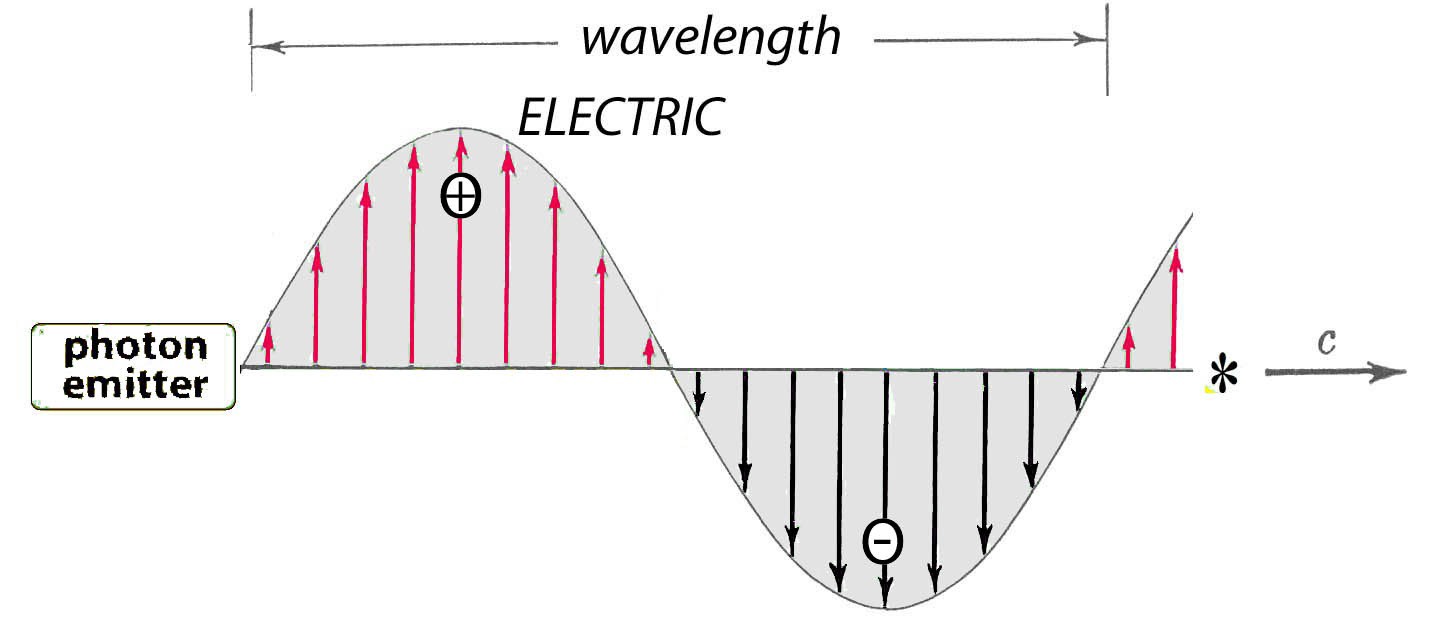
When photons go slower in a medium denser than air, their frequency stays the same.
Their wavelength shortens because they travel a shorter distance during one cycle of vibration. They do not loss energy when slowed, because that is determined by frequency (page 2).
Nor do they gain energy when they speed up on leaving a denser medium.
