Part 1: Introductory Concepts in Planetary Health
2 Introduction to One Health
Learning Objectives
After reading this chapter the learner should be able to:
- Define One Health
- List the three factors that affect One Health
- Explain environment as a common ground for existence of all life forms
- Elaborate on the common health challenges shared between humans and animals
- Identify the roles of human and veterinary medicine in application of One health in controlling infectious diseases
- Explain the significance of domino effect in planetary health
- Synthesize the One health action plan for a specific case situation
Key Words
Important key words for this chapter include:
- One health
- urbanization
- anthropogenic factors
- environment
- domino effect
- biosphere
- Sentinel
- antimicrobial resistance
- zoonotic disease
Section 1: Defining One Health
The One Health Commission has defined One Health as ‘an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and the environment (ecosystems). In other words, ‘One Health’ is the ‘Health of Many’, which utilizes a pluralistic approach for the well-being of its constituents.
What are the constituents of One Health? The humans, animals (domestic, wild, terrestrial, aquatic), plants, and the larger biosphere which sustains life are closely linked and inter-dependent, constitute One Health. Broadly speaking, One Health is geared into the triad of human health, animal health, and the environment health, which are the pillars of One Health (Figure 1).
Since there are many peripheral factors that influence One Health, there is no single, internationally agreed upon definition of it. The most commonly used definition accepted by the US Centers for Disease Control and Prevention and the One Health Commission is: ‘One Health is defined as a collaborative, multisectoral, and transdisciplinary approach—working at the local, regional, national, and global levels—with the goal of achieving optimal health outcomes recognizing the interconnection between people, animals, plants, and their shared environment’. It forms the basis of the tripartite alliance of World Health Organization (WHO), the Food and Agriculture Organization of the United Nations, and the International Organization for Animal Health (FAO et al. 2008).
In order to increase global awareness of the One Health concept, particularly among students, November 3rd is marked as the ‘One Health Day’. It was initiated in 2016 by the One Health Commission (www.onehealthcommission.org), the One Health Platform Foundation (www.onehealthplatform.com), and the One Health Initiative (http://www.onehealthinitiative.com) to raise awareness through educational events organized around the world.
One Health concept is inherently flexible providing liberty to work across perspectives, species, disciplines, thus appropriating to the Planetary Health. The term ‘One Health’ was in fact conceived by the Wildlife Conservative Society in 2004 when the ‘Manhattan principles’ were laid out to endorse an integrated holistic approach to tackle diseases at the human-animal-environment interface (Cook et al. 2004). The ‘One Health’ approach was first used in 2003–2004 to address the emergence of severe acute respiratory disease (SARS) in early 2003 and subsequently in the spread of highly pathogenic avian influenza H5N1 (FAO et al. 2008). The resilience of the concept is vital to its action to diffuse into several inter-related sub-disciplines of science, sociology, and economics in adopting a collaborative action plan for the efficient use of resources and productive outcomes (See Highlight Box A: Focus of One Health).
Planetary health was seeded in us through elementary science chapters of ecosystems, food web and predator-prey relationships. However, when zooming out of the elementary level, One Health may appear very complex. Through this chapter, we will try to understand health from a biological perspective.
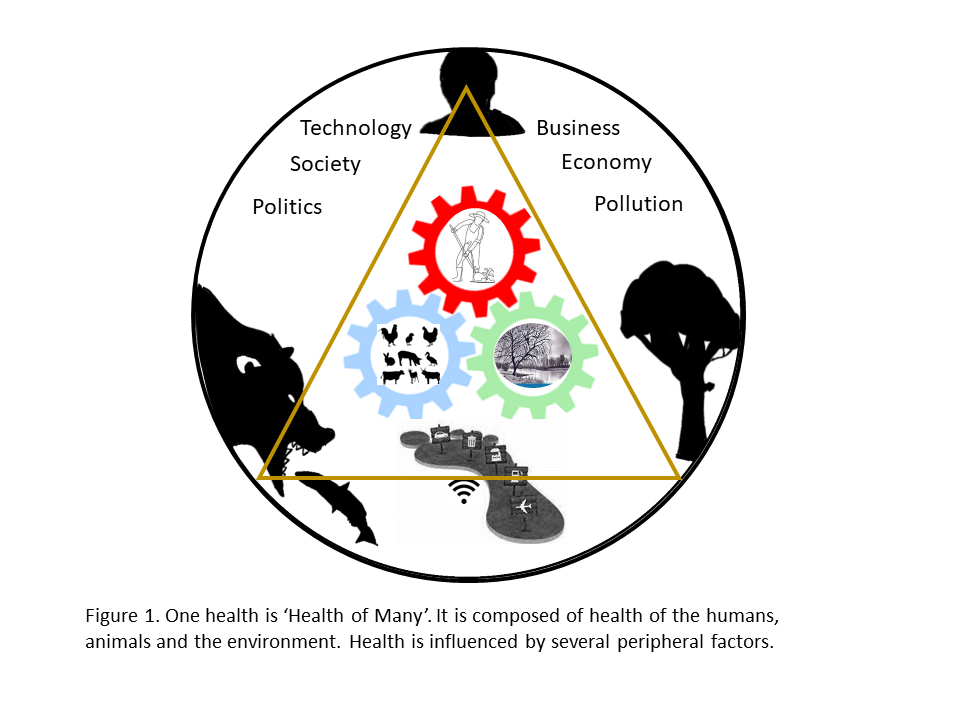
Humans, Animals, and The Environment
Health co-exists in the same space, at the same time
The environment is the most dynamic one, and therefore is the most impactful constituent of the One Health triad. Fundamentally, the environment affects how organisms live, thrive and interact, and thus must be duly considered to achieve optimal health for people and animals (Maller, 2008; Christensen, 2012). The environment can be defined as the amalgamation of ‘the physical, chemical and biological factors’ that determine the growth and survival of all life forms.” (Christensen, 2012). This definition incorporates many different contexts ranging from a local environment, to social environments and the climate in which we exist. As such, the environment can be defined by both the man-made environment, such as urban systems, and the unmodified, natural ecosystems. An ecosystem is composed of all the species, their physical and chemical environment within a specific geographic area (Chu, 1994; Christensen, 2012). The diverse animal and plant species are the ‘eco-stabilizers’ of the planet, which directly and indirectly influence human health.
Animals are inherent entity of our planet. Diverse species of vertebrates and invertebrates promote healthy and sustainable ecosystems. Animals are enrolled in food, income in remote communities, mental health, agriculture, soil fertility, defense services, and scientific research. Animals tend to maintain the homeostasis of ecosystem through intricate web of a ‘prey-predator’ relationship. An example of service of bats to health and economy is by consuming insect pests, which not only saves pesticides cost, but also the health of humans and environment in curbing the use of chemicals (Benjamin, 2021).
Biologically, humans are one of the species among the mammoth catalog of various species on the planet Earth. However, humans created Anthropocene beginning in the 20th century, which is the geological age of the people, by the people and for the people with utmost influence on the whole biosphere (Stephen, 2020). An increase in human population, consumption, faster global movements of biota and abiota, carbon pollution, extinction of animal species and more, created new landscapes to generate new, unforeseen health risks of the planet.
The health systems have conventionally focused on disease surveillance in humans, rather than the source of pathogens. Yet, the majority of human pathogens have originated from animals (“zoonotic” diseases) (Taylor et al. 2001), with 70% of emerging infectious diseases coming from wildlife (Jones et al. 2008). Thus, humans and animals share infectious organisms. The industrial revolution of 20th century soon observed anthropogenic effects in the emergence of several microbial infections, of which Ebola, influenza and Severe Acute Respiratory Syndrome (SARS) viral epidemics in the Urban dwellings have been the wake up calls. Several of such outbreaks have been linked to human practices that lead to the biodiversity loss. Analyses of recently emerging infectious diseases show that anthropogenic factors including, land use change (e.g. deforestation, mining, oil extraction, etc.), food production changes, intensive livestock production, and global trade and travel are among the leading causes of disease emergence (Karesh et al. 2012). Besides, massive prophylactic antimicrobial use in livestock industry for growth promotion, in plant agriculture, as well as inadequate prescribing in companion animal medicine (Kakkar et al., 2017; Laura Kahn, 2017) have driven another set of epidemic in the 21st century, ‘an epidemic of antimicrobial resistant (AMR) organisms’ (O’Neill, 2016; Kakkar et al., 2017).
These practices have caused fundamental changes in the environment, such as (1) change in the basal temperature of the planet Earth, (2) the loss and disruptions of wildlife habitats, and (3) the chemical contamination at the micro-environment level with the spills of antibiotics and industrial sewage. The burden of antibiotic resistance has been ignored and not understood in the environment. Environmental bacteria are the most abundant bacteria, which serve as reservoirs of resistance genes that can become incorporated into human and animal pathogens over time (Kozak et al. 2009; Larsen et al. 2015; Essack, 2018). Such disturbances facilitated emergence and re-emergence of diseases and antimicrobial resistance among pathogens.
Environment is a vacillating factor that has gained greatest attention in terms of climate change. The breach in the environmental integrity has affected ecosystems of pathogens, lifecycle changes in vectors and reservoirs (Essack, 2018 Lancet). Globally, more than 1 billion infections and 1 million deaths annually are attributable to zoonoses, and vector-borne diseases that result in health and socioeconomic burdens (Karesh 2012). Strong evidence suggests that in many vector-borne disease systems, presence of more diverse species helps to reduce the risk of infection (Keesing et al. 2010; LoGiudice et al. 2003). This is due to ‘the dilution effect’, which works through incompetent reservoir hosts that act as barriers by “diluting” the possibility of disease transmission among vectors and competent hosts (Schmidt et al. 2001; Johnson et al. 2008; Keesing et al. 2006; Begon, 2008). In the classic example of Lyme disease, Borrelia pathogen would circulate among a greater proportion of poor reservoirs species of forest mammals with higher levels of biodiversity, thus curbing the infection risk to humans and dogs (Barrett and Osofsky, 2013). Such pattern has also been seen in other vector-borne disease transmissions, e.g., West Nile Virus (WNV), leishmaniasis, and Rocky Mountain spotted fever (Chivian, 2004). The changes in climate and ecosystem has permitted species to expand their range and/or become established in new areas when introduced, as observed with the introduction and establishment of WNV in the United States in 1999, followed by its presence in all of the continental states (Hadler et al. 2015). Similarly, while most cases of Chagas disease documented in USA have been thought to be imported; however, recent detection of Trypanosoma cruzi infections in Texas suggests that endemic transmission within Southern states may be underdiagnosed (Garcia et al. 2015). Similarly, the first detection of rodent-borne Hantavirus pulmonary syndrome in 1993 in United States sustained. The disease re-surfaced in large number of human cases in 2017, of which, 36% were fatal (CDC). Cases of hantavirus pulmonary syndrome have also occurred in Canada, mostly in the western provinces, and in South Americas with large outbreaks typically being linked to changes in environmental factors (Drebot et al., 2015; CDC). Tick-borne diseases are an increasing public health threat in the North America, with a quadrupling incidence of tick-borne ehrlichiosis since 2000 and a steady expansion in the rates of Lyme disease (Heitman et al. 2016; Kugeler et al. 2015).
Despite both endemic and emerging disease risks, actions to mitigate the same remain limited. While animals are the sentinels for environmental contamination, sentinel surveillance is generally underused, and when it does occur, it is poorly utilized due to inter-agency incoordination (Rabinowitz and Conti 2010). In a potential infection of Borrelia in a pet dog, a critical role of veterinary clinician comes into play, since this one case becomes the ‘Sentinel case’ to blow the whistle of an increased wildlife activity in a particular geographic region. The ‘caution’ of potential risk of arising human cases of Lyme disease needs to be communicated promptly across the human medicine interface (Rabinowitz and Conti 2010). At the same time, public health agency should be able to accept the information to attune the clinicians.
Disturbance in ecosystem
To understand the consequences of disturbing interfaces of One Health, here we take an example of the SARS-CoV-2 (COVID-19, begins in December 2019) pandemic. The disturbance of one entity can conduit a ripple effect or a domino effect of disturbances to another (Figure 2). The WHO report suggests that an intermediate animal, possibly the one sold at markets in Wuhan, China passed SARS-CoV-2 to humans after becoming infected with a predecessor coronavirus in bats (Maxmen, 2021). Extensive industrialization and urbanization led to deforestation, thus disturbing the bats dwellings in the wild. The bat population, which is the reservoir of many zoonotic pathogens encroach urban areas, come in contact with animals and infect them; thus passively infecting humans through live and/or dead animal markets leading to an epidemic. The epidemic briskly molts into a pandemic, not giving enough time to even assimilate the chronology of events! Once the outbreak is in full swing, it leads to anthropozoonosis in companion animals (Klaus, 2021) and more so importantly in mink that leads to mass culling of the furry animals, eventually shutting down the mink farming industry in Europe and North America causing severe economic losses (Fenollar, 2021).
What kind of health are we talking about? These events lead to the loss of physical health, socio-economic health, emotional health…the list is on. That’s how One Health is so dynamic, it is affected at the both macro and micro level.
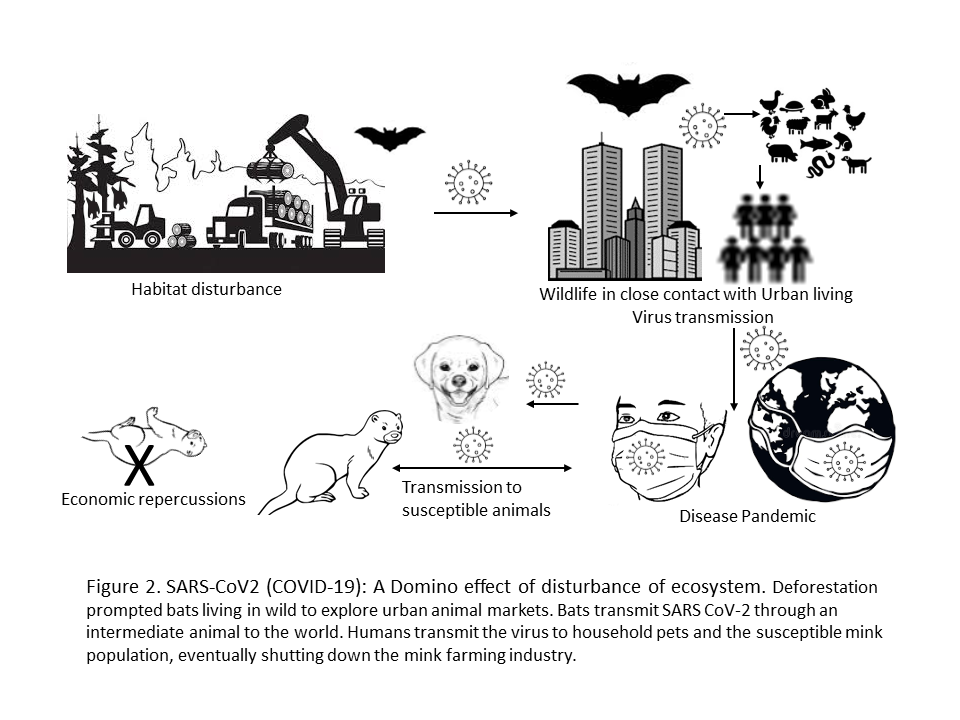
What is the relationship to human health? (acute and chronic)
In general, a veterinary clinician is fairly attuned to the intertwined health of the three pillars of One Health. A veterinarian could identify a zoonotic risk and will counsel the client on the risk of contacting Salmonella from a reptile pet. A human physician will treat Salmonella infection as an acute risk to the human health, but could miss the source. There are several ubiquitous zoonotic pathogens that survive for prolong period in the environment to cause acute and chronic diseases in humans. To name a few here are, Mycobacterium tuberculosis, non-tubercle Mycobacterium species, Coxiella burnetii, Bacillus anthracis and Brucella species that are carried and amplified in animals, and contaminate the environment. Many of these are occupational hazards for people in livestock agriculture, such as veterinarians and the farmers. Some of these, such as Mycobacterium spp, Coxiella burnetii and Brucella spp. are excreted in milk, which discourages consumption of raw milk and highlights food safety. However, this very clause of food safety might not align with many nutritionists and people who benefit from raw milk (Lucey, 2015). Thus, One Health is dynamic across perspectives.
To understand the ‘acute and chronic’ relationship of One Health to human health, a robust example of the largest ever Q fever outbreak in the Netherlands over three years period (2007 to 2009) is shown in Figure 3 and described in Table 2. In the period from March to June 2007, six patients were hospitalized with atypical pneumonia, high fever, headache and dizziness in the province of Noord-Brabant (NB). In May, 2007, family physicians in a rural village of Herpen in the NB province alerted the regional public health service due to an unusually high number of atypical pneumonia in adult patients. These cases were initialy attributed to Mycoplama pneumoniae, but additional serological tests rapidly identified an outbreak of acute Q fever. This notifiable disease was until then very rare in the Netherlands. Despite the implementation of measures aiming at identifying and controlling the source of infection, the number of acute Q fever cases increased enormously to overwhelm the Dutch healthcare. The seasonality of cases followed that of small ruminant (goats and sheep) birth period, however the Human- Veterinary interface remained unseen (Roest et al. 2011). Q fever (or query fever) is caused by a gram negative intracellular bacterium, Coxiella burnetii. The organism is carried asymptomatically by ruminant animals, especially goats and sheep. It is excreted in large numbers in the birthing tissues of placenta and fluids during parturition and abortions to contaminate soil and the environment. C. burnetii is considered an infection risk to the agriculture personnel and potential risk in urban settings as well, since its sporulated form can survive for more than an year in the environment and can be carried for miles with wind (Roest et al. 2011).
Back to the small village of Herpen where a large and protracted human Q fever epidemic started. In 2007, a total of 168 cases were notified, followed by 1,000 notified cases in 2008, mainly in residents of the province of Noord-Brabant. In 2009, a total of 2,354 cases were notified as the epidemic further expanded in neighboring provinces. The clue to the source of infection was nowhere since the communication between the two health sectors could not be interfaced. However, based on the history of visiting goat farms and the proximity of nearby dairy goat farms to the residential addresses of cases indicated the source of infection. During the period, abortion waves due to C. burnetii were confirmed on several dairy goat farms and few dairy sheep farms. The Dutch Q fever epidemic emerged as an important nationwide human and veterinary public health challenge and gained worldwide attention due to its size, disease burden and high societal costs.
The most important lesson learnt from the Dutch Q fever epidemic is that a close cooperation between the human and veterinary fields is essential for responding to outbreaks of zoonotic diseases. The involvement of two different ministries in this Q fever epidemic demonstrated key organizational differences in response structures, with a highly centralized veterinary domain and a strongly decentralized operational public health response. The prerequisite of a ‘One Health’ approach was one of the conclusions made by the official Q fever outbreak evaluation committee in 2010 that evaluated the process and actions of the Dutch government with respect to the Q fever crisis (Roest et al. 2011).
The chronic consequences were examined over the next 14 months post the Q fever outbreak. During the epidemic, over 4,000 cases of acute Q fever were reported, while 215 chronic cases were catalogued (Kampascher, 2014). Vascular focus and endocarditis were the chronic sequalae in 75% of acute Q fever infections. Mortality rate of 9.3% was reported among endocarditis patients and 18.0% in vascular chronic Q fever cases.
In context of Q fever in Atlantic Canada, a characteristic community acquired C. burnetii infection in humans from companion animals is notable. Q fever is endemic in the Atlantic provinces where dogs and cats are the reservoirs of the pathogen, which is shed at the time of parturition. Cases of acute and chronic Q fever in humans with history of contact with parturient cats have been emphasized during 1980’s, and later in a study on the seroprevalence of C. burnetii antibodies in patients during a period of 2004 to 2007 (Marrie et al. 2008).
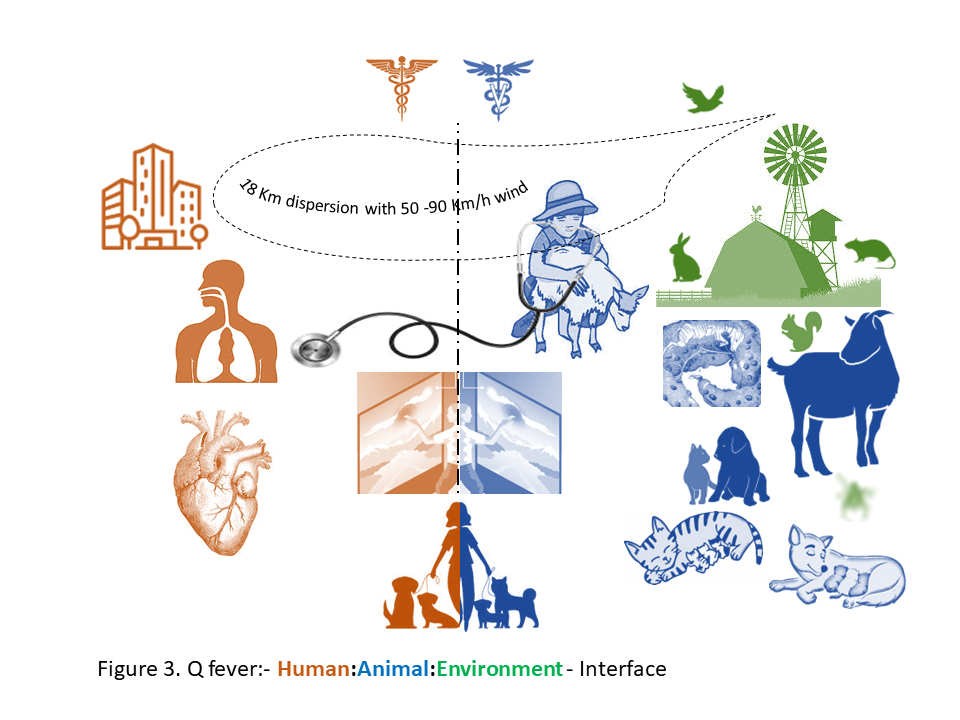
Cross-talks to Co-exist
It is needed to emphasize here that the stage of Cross-talks and relationships among the constituents of One Health was set during the primordial times when the planet Earth began upholding life. Humans tamed animals to develop civilizations with them, explored and inhabited deserted parts of the Earth. The socio-cultural habits influenced the gearing of the triad. For example, in some culture practices, several animals and plants are considered as sacred. This very notion pulls away the dominion of human over Earth’s resources, thus aid in saving medicinal plants and directs the attitude towards health and wellness of all (Barrett and Osofsky, 2013; Stephen, 2020). When we began to equate water to H2O, the very significance and naturalism of water element deteriorated leading to anthropocentric water managements, the consequences of which are the adverse effects on the ecosystems which the naturalists and philosophers argue (Blackstock, 2001). The gaps in the triad began in the past two centuries due to anthropogenic pressures, thereby ignoring the big picture, ‘the Circle of Life’. Humans are in fact realizing it in the 21st century as the disturbances of the ecosystem are reciprocating upfront to face the ‘shared risk’. The common denominator here is the Earth’s biosphere, which is adversely affected due to over-exploitation of resources. Thus, in the last two decades, the way forward paved is the coming together of people of diverse disciplines and programs to embrace the fact that the ‘health is the interactions and inter-dependence among animals, humans and the environments’ (Highlight Box B: Notes for Clinicians). The programs and policies at the international level and Government levels are bringing in new collaborations and thought process to refrain from limiting health to a ‘mathematical data point’; instead, understand health as a ‘positive socio-ecological’ phenomenon.
Implementation of One Health is inadequate on the ground. It faces ongoing barriers of professional segregation and data sharing in the animal and human health communities, which downsize relevant evidences to blank a clear understanding of the significance of animal and environmental health indicators to human health, which in-turn leads to limited acceptance of sentinel surveillance approaches. The constant efforts toward systematic and sustained holistic action is the key to achieve the goal of One Health and Earth stewardship. You may be at various places at a given time and space, such as, in your residential neighborhood with pets, at the beach, hiking along the marshy areas, visiting a hospital or an animal farm; being the health care providers, from now onwards, think of ‘One Health’ around you.
| Table 1. Dynamics of One Health: Q-fever epidemic | |
| Lesson: Close cooperation between the human and veterinary fields: Clinicians and the Government Ministries | |
Human
|
Dairy goats
|
| One Health: In Action: Harm reduction, Health promotion | |
| Human
Response: Follow-up chronic Q fever Sero-surveillance |
Animal, Environment
Response:
|
Highlight Box A: Focus of One Health
Consequences, responses, and actions at the animal–human–environment interfaces
Aim: Healthy biosphere of sustainable development with Zero impact
-
Emerging and endemic zoonoses
-
Antimicrobial resistance (AMR)
-
Health of food animals
-
Food safety
-
Risks and benefits of companion animals
-
Wildlife health
-
Environment conservation
-
Socioeconomic impact
- Resources: efficient use, devoid of exploitation
-
Education, communication
Highlight Box B: Notes for Clinician
- The majority of human infectious diseases originated from animals
- Nearly ¾ of recently-emerging diseases originating from wildlife
- Emerging diseases pose threats to public health, food security and endangered species
- Growing global human population, and anthropogenic factors are drivers of biodiversity loss
- Loss of 60% of the essential ecosystem services of the planet
- Most public health systems lack integrated mechanisms to adequately detect and respond to infectious threats
- Knowledge of pathogens in wildlife can guide risk prioritization and prognostic modeling of outbreaks
- Think and Act One Health
Section 2: Animal Models in Medical Research
Learning Objectives For Chapter 2 Section 2
- After reading this chapter you should be able to:
- Define the phases of the medical research pipeline
- Describe, providing examples, how animals contribute to medicine
- Provide examples that illustrate the 3 R’s of animal based research
Key Words
Keywords associated with this chapter include:
- Discovery
- Translational research
- Animal models
- Ethical animal research
- The three R’s
Most people don’t know that virtually all medical treatment and procedures have had some basis in learning about disease and how to treat it through research and teaching. Because humans share a genetic heritage with all living beings on the planet, it is not unusual that a biological phenomenon discovered in a simple organism can serve as the foundation for development of a treatment for cancer, heart disease, infection or neurological conditions. Research that utilizes non-human living organisms is a major aspect of biomedical research, and this benefit to humanity must be received with respect and humility.
The research pipeline
The pharmaceutical – biotechnology industry has defined a three phase research approach to delivery of new products to the therapy armamentaria of the various clinical specialties. The pipeline involes 3 phases: Discovery, translational, and clinical
Evidence and proof of efficacy of a new treatment for patients must pass strict regulatory requirements, and these data most often requires animal research. In the pre-clinical phases, scientific investigation that is designed to understand the fundamental aspects of physiological and pathological phenomena is referred to as discovery research and can involve in vitro cell systems or in vivo animal models. In the course of finding out new things, there may be opportunities to apply these findings in a manner and in systems that point towards the possibility of application to medical diagnosis and treatment. These secondary, translational studies usually involve animal models because they test the possibility of the application to a whole-body system. Once the translational data show promising findings, the application can then be tested in the human setting, in clinical trials.
Why animals are used in human medical research
Animals are used in both discovery and translational research, but they are also used for clinical trials for veterinary applications. There is evidence that since the beginning of medicine, animals have been used to test theories and new treatments and therapies. The choice of the animal model system is critical, because although humans and animals superficially may share similar physiological and genetic properties, the response to application of a new drug, or therapy can very possibly be quite different between species. The hypothesis is that humans and animals share the same or similar physiological processes and genetic heritage, so they likely respond similarly to therapies developed by medical research.
Examples of Nobel Prize winning medical discoveries that have benefitted from animal research
Many people think that medical discoveries have come from studies on larger mammals such as primates. In fact, most of the fundamental discoveries on how the body works have their foundation in studies using what may appear to us as humbler, simple beings. For example, the sea slug, Aplysia is a foot long mollusk that has only 20,000 nerve cells, as compare to billions in the human body. Dr. Carlsson, Greengard and Kandel were awarded the Nobel Prize for working out how neurons communicate in the brain. What was interesting is that their research implied that each person’s memories and experiences are unique, based on the gene expression patterns of neural networks recruited to form those memories. They found evidence to suggest that learning and memory are based on plastic changes in the synaptic contacts in localized regions of the brain. Such knowledge has been essential to understand, diagnose and treat psychiatric and neurological problems in people.
Other “simple” organisms, such as that soil dwelling round worm, Caenorhabditis elegans, have made significant contributions to our understanding of how the body develops. In 2002, Sydney Brenner, H. Robert Horvitz and John E. Sulston won the Nobel prize for their work using nematode worms, on how organs develop in the body by growth and differentiation and by a process called apoptosis, or programmed cell death, to maintain normal homeostasis. This was an important finding not only for basic understanding of human development, but also in our understanding of cancer, in which loss of apoptosis contributes to uncontrolled cell division, leading to tumor formation. Also the first genome ever to be sequenced was the nematode’s, in 1998.
At least 5 Nobel prizes for Physiology or medicine have been awarded for research directly involving the common fruitfly, Drosophila melanogaster. Each of these discoveries has been used as fundamental studies upon which the development of new medical treatments and technologies have been based.
The foundation for biomedical research states that of all 224 Nobel prizes for Medicine or Physiology, 188 have used animal models. Discoveries ranging from understanding how cells divide to the development of magnetic resonance imaging, or MRI, and the use of antibiotics to treat stomach ulcers all have had a basis in animal research. Animal research is also most obviously a key activity for veterinary medicine as well sharing many treatments and diagnostic approaches with the human counterpart. Click on the ABR logo to see the complete list of prize winners and the animal model systems they have used.
The top ten drugs that have used animals for drug testing in human medicine are also listed in the table. Click on the FBR link to see the whole list. In the United States and Canada, animal testing of drugs for pre-clinical trials in humans is required, and as you can see, drugs that are used extensively to manage blood pressure, cholesterol levels and a range of common conditions, have utilized many different species, both mammalian and non-mammalian.
Ethical utilization of animals for medical research
While animal models are an essential part of medical discovery and research, it is necessary to utilize them in an ethically responsible manner. Of most importance is that the loss of animal life and quality of animal life in the generation of biomedical data are minimized as much as possible. Consequently it should be ensured that the ratio of data obtained to animal lives utilized is maximized to ensure that as much as is possible, no individual animal life is taken without sufficient rationale.
For this reason every organization, whether it be universities, research institutions and private companies in Canada and in most countries requires strict regulations regarding the use of animals for research. In Canada, the use of animals in research is regulated by the Canadian Council on Animal Care (https://ccac.ca), or CCAC. There are a series of nine (9) principles (https://ccac.ca/en/standards/fundamental-principles.html) that must be adhered to. The guidelines (https://ccac.ca/en/standards/guidelines/) for each institution were developed in line with these principles. Each institution is inspected regularly to ensure standards are met in order to obtain certification to continue to utilize animals.
The 3 R’s of animal research
Central to the principles of good practice in the utilization of animals for research are– Replacement, refinement and reduction. These concepts were established in the 1960’s by two British biologists, Russell and Burch and formed the foundations for ethical use of animals in research to this very day. This video presents three case studies exemplifying the 3-Rs and scientific value gained from following these principles.
Reduction – refers to the change in an experimental protocol, which reduces the necessity to utilize as many animals as originally intended, while still achieving research objectives. Determining statistically significant minimal sample sizes, for example would contribute to reduction. Careful experimental design utilizing analytical approaches that maximize the collection of data from each subject is another way reduction can be achieved. The objective here is to gather as much data as possible utilizing a minimal number of animals.
Refinement – refers to the application of techniques, or measures that improve the quality of life of animal subjects. An example of this is the application of anaesthetic and analgesic drugs when performing experiments and making certain all pre- and post- procedure conditions are in the interest of the comfort and well-being of the animals. Refinement includes using non-invasive procedures such as MRI, CT and ultrasonography technologies found in human healthcare that have been developed for veterinary and animal research.
Replacement – While animal models are considered the best approach in pre-clinical studies for new drug and treatment applications for humans, it is sometimes possible to utilize methods to gather evidence. Using non-vertebrate species, cell lines from human tissues and computational methods to generate models that can rule out the necessity for spurious or non-contributory experiments all are considered forms of replacement.
Video presentation: The 3R’s – Their definition, application and importance to your work. https://www.youtube.com/watch?v=LdKiD5pW2XY&t=9s
Animal research is an important part of human medicine, and humans depend on animals for healthy living. This video is an interesting and comprehensive presentation of the 3R’s providing excellent examples of how significant discoveries have utilized these principles to obtain excellent research findings that impact on human health care. The video encapsulates everything you need to know about the ethical treatment of animals in research.
References
Barrett MA and Osofsky SA. 2013. One Health: Interdependence of People, Other Species, and the Planet. Ch 30. In: Jekel’s Epidemiology, Biostatistics, Preventive Medicine, and Public Health. Ed. Wild D, Elmore JG, Katz DL and Lucan SC.
Begon M. 2008. Effects of host diversity in disease dynamics. In: Ostfeld RS , Keesing F , Eviner VT , editors: Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems , Princeton, NJ , 2008 , Princeton University Press.
Benjamin, J. 2021. Celebrating the Special Powers of Bats. USDA Public Affairs Specialist in Animals Conservation. https://www.usda.gov/media/blog/2021/10/27/celebrating-special-powers-bats
Blackstock, M. 2001. Water: A First Nations’ spiritual and ecological perspective. B.C. Journal of Ecosystems and Management. Vol 1: 1-14.
Centers for Disease Control and Prevention (CDC). Reported cases of hantavirus infection. Available at: https://www.cdc.gov/hantavirus/surveillance/index.html.
Chivian E , Bernstein AS : Embedded in nature: human health and biodiversity . 2004. Environ Health Perspect 112 : A12 .
Christensen N. 2012. In: The environment and you , Boston, Addison Wesley .
Chu CM and Simpson R. 1994. In: Ecological public health: from vision to practice, Toronto, Centre for Health Promotion, University of Toronto.
Cook, R.A., Karesh, W.B. and Osofsky, S.A. 2004. The Manhattan Principles on ‘One World, One Health’. Conference summary: One World, One Health: Building Interdisciplinary Bridges to Health in a Globalized World, 29 September 2004, New York. Available at: http://www.oneworldonehealth.org/sept2004/owoh_ sept04.html
Drebot MA, Jones S, Grolla A, Safronetz D, Strong JE, Kobinger G, Lindsay RL. 2015. Hantavirus pulmonary syndrome in Canada: An overview of clinical features, diagnostics, epidemiology and prevention. Can Commun Dis Rep. Jun 4;41(6):124-131. doi: 10.14745/ccdr.v41i06a02. PMID: 29769944; PMCID: PMC5864423.
Essack S. Y. 2018. Environment: the neglected component of the One Health triad. The Lancet. Planetary health, 2(6), e238–e239. https://doi.org/10.1016/S2542-5196(18)30124-4.
FAO, OIE and WHO. 2010. The FAO-OIE-WHO Collaboration: Sharing responsibilities and coordinating global activities to address health risks at the animal-human-ecosystems interfaces. Tripartite concept note. Food and Agriculture Organization of the United Nations, World Organisation for Animal Health, World Health Organization, Geneva, Switzerland. Available at: http://www.who.int/influenza/ resources/documents/tripartite_concept_note_ hanoi/en/
Larsen, J., Petersen, A., Sorum, M., Stegger, M., van Alphen, L. et al. 2015. Meticillin-resistant Staphylococcus aureus CC398 is an increasing cause of disease in people with no livestock contact in Denmark, 1999 to 2011. Euro Surveillance 20(37): 30021.
Fenollar, F., Mediannikov, O., Maurin, M., Devaux, C., Colson, P., Levasseur, A., Fournier, P. E., & Raoult, D. 2021. Mink, SARS-CoV-2, and the Human-Animal Interface. Frontiers in microbiology, 12, 663815. https://doi.org/10.3389/fmicb.2021.663815
Garcia MN, Aguillar D, Gorchakov R, et al. 2015. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 92:325–330.
Hadler JL, Patel D, Nasci RS, et al. 2015. Assessment of arbovirus surveillance 13 years after introduction of West Nile virus, United States. Emerg Infect Dis. 21:1159–1166.
Heitman KN, Dahlgren FS, Drexler NA, Massung RF, Behrevesh CB. 2016. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 94:52–60.
Johnson PTJ , Hartson RB , Larson DJ , et al. 2008. Diversity and disease: community structure drives parasite transmission and host fitness . Ecol Lett 11: 1017 – 1026.
Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. & Daszak P. 2008. Global trends in emerging infectious diseases. Nature. 451: 990- U4.
Kahn, LH. 2017. The one-health way The health of animals, humans and the environment will be better served by breaking down barriers. Nature 543:S47
Kakkar, M., Walia, K., Vong, S., Chatterjee, P. and Sharma, A. 2017. Antibiotic resistance and its containment in India. BMJ (Clinical Research edn) 358, j2687.
Kampschreur, L. M., Delsing, C. E., Groenwold, R. H., Wegdam-Blans, M. C., Bleeker-Rovers, C. P., de Jager-Leclercq, M. G., Hoepelman, A. I., van Kasteren, M. E., Buijs, J., Renders, N. H., Nabuurs-Franssen, M. H., Oosterheert, J. J., & Wever, P. C. 2014. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. Journal of clinical microbiology, 52(5), 1637–1643. https://doi.org/10.1128/JCM.03221-13.
Karesh WB, Dobson A, Lloyd-Smith JO, et al. 2012. Ecology of zoonoses: natural and unnatural histories. Lancet. 380:1936–1945.
Keesing F , Holt RD , Ostfeld RS. 2006. In: Effects of species diversity on disease risk . Ecol Lett 9 : 485 – 498.
Keesing F , Belden LK , Daszak P , et al 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases . Nature 468 ( 7324 ): 647 – 652.
Klaus, J., Zini, E., Hartmann, K., Egberink, H., Kipar, A., Bergmann, M., Palizzotto, C., Zhao, S., Rossi, F., Franco, V., Porporato, F., Hofmann-Lehmann, R., & Meli, M. L. 2021. SARS-CoV-2 Infection in Dogs and Cats from Southern Germany and Northern Italy during the First Wave of the COVID-19 Pandemic. Viruses, 13(8), 1453. https://doi.org/10.3390/v13081453.
Kozak, G.K., Boerlin, P., Janecko, N., Reid-Smith, R.J. and Jardine, C. 2009. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Applied and
Environmental Microbiology 75, 559–566.
Kugeler KJ, Farley GM, Forrester JD, Mead P. 2015. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 21:1455–1457.
LoGiudice K , Ostfeld RS , Schmidt KA , et al. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk . Proc Natl Acad Sci USA 100: 567 – 571.
Lucey J. A. 2015. Raw Milk Consumption: Risks and Benefits. Nutrition today, 50(4), 189–193. https://doi.org/10.1097/NT.
Maller C , Townsend M , St Leger L , et al. 2008. In: Healthy parks, healthy people: the health benefits of contact with nature in a park context , ed 2 , Melbourne, Deakin University and Parks Victoria.
Marrie, T. J., Campbell, N., McNeil, S. A., Webster, D., & Hatchette, T. F. 2008. Q fever update, Maritime Canada. Emerging infectious diseases, 14(1), 67–69. https://doi.org/10.3201/eid1401.071256
Maxmen A. 2021. WHO report into COVID pandemic origins zeroes in on animal markets, not labs. Nature, 592(7853), 173–174. https://doi.org/10.1038/d41586-021-00865-8
O’Neill. 2016. Review on Antimicrobial Resistance. Tackling Drug-resistant Infections Globally: Final Report and Recommendations. World Health Organization, Geneva, Switzerland.
Rabinowitz P, Scotch M and Conti L. 2009. Human and animal sentinels for shared health risks. Vet Ital. 45:23–24.
Roest, H. I., Tilburg, J. J., van der Hoek, W., Vellema, P., van Zijderveld, F. G., Klaassen, C. H., & Raoult, D. 2011. The Q fever epidemic in The Netherlands: history, onset, response and reflection. Epidemiology and infection, 139(1), 1–12. https://doi.org/10.1017/S0950268810002268
Schmidt KA and Ostfeld RS. 2001. Biodiversity and the dilution effect in disease ecology . Ecology 82 : 609 – 619.
Stephen, C. 2020. In: Animals, Health, and Society: Health Promotion, Harm Reduction, and Health Equity in a One Health World.
Taylor L.H., Latham S.M. & Woolhouse M.E.J. 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 356: 983-989

